A) a gas is made up of molecules
B) a gas assumes the volume of its container
C) a gas may consist of both elements and compounds
D) gases are always mixtures
E) All of the above answers are correct.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The average kinetic energy of the particles of a gas is directly proportional to __________.
A) the rms speed
B) the square of the rms speed
C) the square root of the rms speed
D) the square of the particle mass
E) the particle mass
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The temperature of a sample of CH4 gas (10.34 g) in a 50.0 L vessel at 1.33 atm is __________ °C.
A) 984
B) -195
C) 195
D) 1260
E) -1260
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The effusion rate of a gas is proportional to the square root of its molar mass.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following, __________ is a correct statement of Boyle's law.
A) PV = constant
B) ![]() = constant
= constant
C) ![]() = constant
= constant
D) ![]() = constant
= constant
E) ![]() = constant
= constant
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is a valid statement of Avogadro's law?
A) ![]() = constant
= constant
B) ![]() = constant
= constant
C) PV = constant
D) V = constant × n
E) V = constant × P
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Given the equation: C2H6 (g)+ O2 (g)→ CO2 (g)+ H2O (g)(not balanced) Determine the number of liters of O2 consumed at STP when 270.0 grams of C2H6 is burned.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following gases would have the highest average molecular speed at 25°C?
A) O2
B) N2
C) CO2
D) CH4
E) SF6
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Gaseous mixtures __________.
A) can only contain molecules
B) are all heterogeneous
C) can only contain isolated atoms
D) are all homogeneous
E) must contain both isolated atoms and molecules
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following gases would deviate the least from ideal gas behavior?
A) Ne
B) CH3Cl
C) Kr
D) CO2
E) F2
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas vessel is attached to an open-end manometer filled with a nonvolatile liquid of density 0.993 g/mL as shown below. 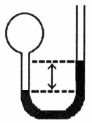 The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is __________ atm.
The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is __________ atm.
A) 1.05
B) 1.01
C) 0.976
D) 0.993
E) 1.08
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
If the temperature is lowered from 60°C to 30°C, the volume of a fixed amount of gas will be one half the original volume.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas originally at 27°C and 1.00 atm pressure in a 3.9 L flask is cooled at constant pressure until the temperature is 11°C. The new volume of the gas is __________ L.
A) 0.27
B) 3.7
C) 3.9
D) 4.1
E) 0.24
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas mixture of Ne and Ar has a total pressure of 4.00 atm and contains 16.0 mol of gas. If the partial pressure of Ne is 2.75 atm, how many moles of Ar are in the mixture?
A) 11.0
B) 5.00
C) 6.75
D) 9.25
E) 12.0
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pressure of a sample of CH4 gas (6.022 g) in a 30.0 L vessel at 402 K is __________ atm.
A) 2.42
B) 6.62
C) 0.413
D) 12.4
E) 22.4
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following equations shows an incorrect relationship between pressures given in terms of different units?
A) 1.20 atm = 122 kPa
B) 152 mm Hg = 2.03 × 104Pa
C) 0.760 atm = 578 mm Hg
D) 1.0 torr = 2.00 mm Hg
E) 1.00 atm = 760 torr
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The density of NO2 in a 4.50 L tank at 760.0 torr and 25.0°C is __________ g/L.
A) 1.64
B) 9.30
C) 1.68
D) 1.88
E) 3.27
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas at a pressure of 10.0 Pa exerts a force of __________ N on an area of 5.5 m2.
A) 55
B) 0.55
C) 5.5
D) 1.8
E) 18
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of a sample of gas (2.49 g) was 752 mL at 1.98 atm and 62°C. The gas is __________.
A) SO2
B) SO3
C) NH3
D) NO2
E) Ne
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
The deviation from ideal behavior of a gas is most evident at __________ and/or low temperature.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 172
Related Exams