A) 1 amp ∙ s
B) 1 J/s
C) 96485 C
D) 1 J/C
E) 1 C/J
G) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
At constant temperature and pressure the Gibbs free energy value is a measure of the ________ of a process.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many seconds are required to produce 1.0 g of silver metal by the electrolysis of a AgNO3 solution using a current of 60 amps?
A) 5.4 × 104
B) 3.2 × 103
C) 15
D) 3.7 × 10-5
E) 30
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The standard cell potential (E° cell) for the reaction below is +1.10 V. The cell potential for this reaction is ________ V when the concentration of [Cu2+] = 1.0 × 10-5 M and [Zn2+] = 3.0 M. Zn (s) + Cu2+ (aq) → Cu (s) + Zn2+ (aq)
A) 1.42
B) 1.26
C) 0.94
D) 0.78
E) 1.10
G) A) and C)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
The purpose of the salt bridge in an electrochemical cell is to ________.
A) maintain electrical neutrality in the half-cells via migration of ions
B) provide a source of ions to react at the anode and cathode
C) provide oxygen to facilitate oxidation at the anode
D) provide a means for electrons to travel from the anode to the cathode
E) provide a means for electrons to travel from the cathode to the anode
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Table 20.2 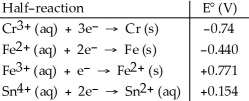 -The standard cell potential (E°cell) for the voltaic cell based on the reaction below is ________ V. 2Cr (s) + 3Fe2+ (aq) → 3Fe (s) + 2Cr3+ (aq)
-The standard cell potential (E°cell) for the voltaic cell based on the reaction below is ________ V. 2Cr (s) + 3Fe2+ (aq) → 3Fe (s) + 2Cr3+ (aq)
A) +0.30
B) +2.80
C) +3.10
D) +0.83
E) -0.16
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The standard emf for the cell using the overall cell reaction below is +2.20 V: 2Al (s) + 3I2 (s) → 2Al3+ (aq) + 6I- (aq) The emf generated by the cell when [Al3+] = 3.5 × 10-3 M and [I-] = 0.30 M is ________ V.
A) 2.20
B) 2.28
C) 2.12
D) 2.36
E) 2.23
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element is reduced in the reaction below? I- + MnO4- + H+ → I2 + MnO2 + H2O
A) I
B) Mn
C) O
D) H
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which substance is the oxidizing agent in the reaction below? Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
A) Pb
B) H2SO4
C) PbO2
D) PbSO4
E) H2O
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Table 20.2 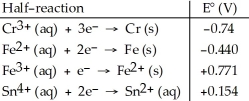 -Which of the following reactions will occur spontaneously as written?
-Which of the following reactions will occur spontaneously as written?
A) Sn4+ (aq) + Fe3+ (aq) → Sn2+ (aq) + Fe2+ (aq)
B) 3Fe (s) + 2Cr3+ (aq) → 2Cr (s) + 3Fe2+ (aq)
C) Sn4+ (aq) + Fe2+ (aq) → Sn2+ (aq) + Fe (s)
D) 3Sn4+ (aq) + 2Cr (s) → 2Cr3+ (aq) + 3Sn2+ (aq)
E) 3Fe2+ (aq) → Fe (s) + 2Fe3+ (aq)
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many minutes will it take to plate out 4.56 g of Ni metal from a solution of Ni2+ using a current of 50.5 amps in an electrolytic cell?
A) 2.47
B) 4.95
C) 4.55
D) 148
E) 297
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the coefficient of the permanganate ion when the following equation is balanced? MnO4- + Br- → Mn2+ + Br2 (acidic solution)
A) 1
B) 2
C) 3
D) 5
E) 4
G) A) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
The standard cell potential (E°cell) of the reaction below is +1.34 V. The value of ΔG° for the reaction is ________ kJ/mol. 3 Cu (s) + 2 MnO4- (aq) + 8H+ (aq) → 3 Cu2+ (aq) + 2 MnO2 (s) + 4 H2O (l)
A) -24.3
B) +259
C) -259
D) +776
E) -776
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which transformation could take place at the cathode of an electrochemical cell?
A) MnO2 → MnO4-
B) Br2 → BrO3-
C) NO → HNO2
D) HSO4- → H2SO3
E) Mn2+ → MnO4-
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which substance is the reducing agent in the reaction below? Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
A) Pb
B) H2SO4
C) PbO2
D) PbSO4
E) H2O
G) A) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which substance is the oxidizing agent in the following reaction? Fe2S3 + 12HNO3 → 2Fe(NO3) 3 + 3S + 6NO2 + 6H2O
A) HNO3
B) S
C) NO2
D) Fe2S3
E) H2O
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider an electrochemical cell based on the reaction: 2H+ (aq) + Sn (s) → Sn2+ (aq) + H2 (g) Which of the following actions would not change the measured cell potential?
A) lowering the pH in the cathode compartment
B) addition of more tin metal to the anode compartment
C) increasing the tin (II) ion concentration in the anode compartment
D) increasing the pressure of hydrogen gas in the cathode compartment
E) Any of the above will change the measured cell potential.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a voltaic cell, electrons flow from the ________ to the ________.
A) salt bride, anode
B) anode, salt bridge
C) cathode, anode
D) salt bridge, cathode
E) anode, cathode
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which transformation could take place at the anode of an electrochemical cell?
A) Cr2O72- → Cr2+
B) F2 to F-
C) O2 to H2O
D) HAsO2 to As
E) None of the above could take place at the anode.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number of manganese in MnO2?
A) +3
B) +2
C) +1
D) +4
E) +7
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 114
Related Exams