A) H+(aq) + OH-(aq) H2O(aq)
B) H+(aq) + CH3NH2(aq) CH3NH3+(aq)
C) OH-(aq) + HCN(aq) H2O(aq) + CN-(aq)
D) HCN(aq) + CH3NH2(aq) CH3NH3+(aq) + CN-(aq)
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the direction in which the equilibrium will lie for the reaction H2SO3(aq) + HCO3- (aq) 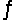 HSO3-(aq) + H2CO3(aq) .
Ka1(H2SO3) = 1 * 10-2; Ka1(H2CO3) = 4.2 * 10-7
HSO3-(aq) + H2CO3(aq) .
Ka1(H2SO3) = 1 * 10-2; Ka1(H2CO3) = 4.2 * 10-7
A) to the right
B) to the left
C) in the middle
E) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
Rain collected on a remote island in the Pacific assumed to be unaffected by human pollution.The pH of the rainwater on this island was not 7.Do you expect the pH to be greater than 7 or less than 7? Explain your reasoning.
Correct Answer

verified
The pH is expected to be less ...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Will a 0.1 M solution of NaH2PO4(aq)be acidic, basic, or neutral?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of these net ionic equations represents the reaction of a strong acid with a weak base?
A) H+(aq) + OH-(aq) H2O(aq)
B) H+(aq) + CH3NH2(aq) CH3NH3+(aq)
C) OH-(aq) + HCN(aq) H2O(aq) + CN-(aq)
D) HCN(aq) + CH3NH2(aq) CH3NH3+ (aq) + CN-(aq)
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suppose that ammonia, applied to a field as a fertilizer, is washed into a farm pond containing 3.0 * 106 L of water. If the pH of this pond is found to be 9.81, what volume of liquid ammonia found its way into the pond? [Given: Kb(NH3) = 1.8 * 10-5; the density of liquid ammonia is 0.771 g/cm3]
A) 15 L
B) 25 L
C) 11 L
D) 19 L
E) 320 L
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
In the reaction Ag+(aq)+ Cl-(aq) AgCl(s), Ag+ acts as a Lewis acid.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In 0.10 M KCN, the chemical species with the highest concentration (except H2O) is
A) Na+
B) CN-
C) H3O+ (or H+)
D) OH-
E) K+
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the acids HBr, H2Se, and H3As in order of increasing acid strength.
A) HBr < H2Se < H3As
B) HBr < H3As < H2Se
C) H2Se < H3As < HBr
D) H3As < H2Se < HBr
E) H3As < HBr < H2Se
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pH of a 0.10 M HCN solution that is 0.0070% ionized.
A) 1.00
B) 0.00070
C) 3.15
D) 5.15
E) 7.00
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the pH of an acid rain storm is approximately 3.0, how many times greater is the [H+] in the rain than in a cup of coffee having a pH of 5.0?
A) 1000
B) 100
C) 20
D) 1.7
E) 0.60
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH of a certain solution is 3.0. How many H+(aq) ions are there in 1.0 L of the solution?
A) 0.001 ions
B) 1,000 ions
C) 6.* 1020 ions
D) 3 ions
E) 6.* 1026 ions
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of an aqueous solution that contains 2.7 *1020 H3O+ ions per liter of solution?
A) 2.70
B) 3.35
C) 6.02
D) 10.65
E) 11.30
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which response gives the products of hydrolysis of KF?
A) KOH + HF
B) OH- + HF
C) KOH + H+ + F-
D) KH + F- + OH-
E) No hydrolysis occurs.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the pH of a KOH solution made by mixing 0.251 g KOH with enough water to make 1.00 * 102 mL of solution.
A) 1.35
B) 2.35
C) 7.00
D) 11.65
E) 12.65
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Write the chemical formula for hydrochloric acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For H3PO4, Ka1 = 7.3 * 10-3, Ka2 = 6.2 *10-6, and Ka3 = 4.8 *10-13. An aqueous solution of Na3PO4 therefore would be
A) neutral
B) basic
C) acidic
E) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pH of a 3.5 * 10-3 M HNO3 solution.
A) -2.46
B) 0.54
C) 2.46
D) 3.00
E) 3.46
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration of H+ in a 2.5 M HCl solution?
A) 0
B) 1.3 M
C) 2.5 M
D) 5.0 M
E) 10.M
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
When 2.0 * 10-2 mole of nicotinic acid (a monoprotic acid)is dissolved in 350.mL of water, the pH is 3.05.What is the Ka of nicotinic acid?
Correct Answer

verified
1.4 * 10View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 81 - 100 of 163
Related Exams