A) hydropotassium phosphate.
B) potassium dihydrogen phosphate.
C) potassium diphosphate.
D) potassium hydrogen(II) phosphate.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which subatomic particle has the smallest mass?
A) a proton
B) a neutron
C) an electron
D) an alpha particle
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following drawings,shaded spheres represent cations and unshaded spheres represent anions. 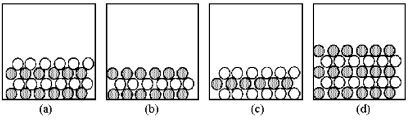 -Which drawing represents the ionic compound CaCl2?
-Which drawing represents the ionic compound CaCl2?
A) drawing (a)
B) drawing (b)
C) drawing (c)
D) drawing (d)
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If shaded and unshaded spheres represent atoms of different elements,as shown in drawing (1) ,which drawings (2) -(4) represent the law of multiple proportions? 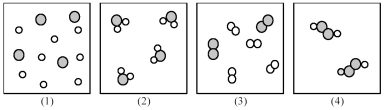
A) only drawings (2) and (3)
B) only drawings (2) and (4)
C) only drawings (3) and (4)
D) drawings (2) ,(3) ,and (4)
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of pure lithium carbonate contains 18.8% lithium by mass.What is the % lithium by mass in a sample of pure lithium carbonate that has twice the mass of the first sample?
A) 9) 40%
B) 18.8%
C) 37.6%
D) 75.2%
F) All of the above
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
What is the chemical formula for magnesium hydride?
A) MgH2
B) MgOH
C) MgOH2
D) Mg(OH) 2
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the identity of element Q if the ion Q2+ contains 10 electrons?
A) C
B) O
C) Ne
D) Mg
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of pure calcium fluoride with a mass of 15.0 g contains 7.70 g of calcium.How much calcium is contained in 40.0 g of calcium fluoride?
A) 2) 27 g
B) 7) 70 g
C) 15.0 g
D) 20.5 g
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Methane and oxygen react to form carbon dioxide and water.What mass of water is formed if 3.2 g of methane reacts with 12.8 g of oxygen to produce 8.8 g of carbon dioxide?
A) 7) 2 g
B) 8) 8 g
C) 14.8 g
D) 16.0 g
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
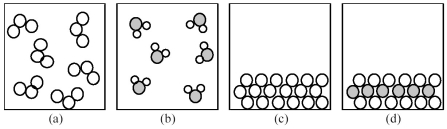 -If shaded and unshaded spheres represent atoms of different elements,which of the above drawings most likely represents an ionic compound at room temperature and a pressure of 1 atm?
-If shaded and unshaded spheres represent atoms of different elements,which of the above drawings most likely represents an ionic compound at room temperature and a pressure of 1 atm?
A) drawing (a)
B) drawing (b)
C) drawing (c)
D) drawing (d)
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Apple juice is an example of
A) a compound.
B) an element.
C) a heterogeneous mixture.
D) a homogeneous mixture.
F) B) and D)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Which of the following statements about gamma radiation is false?
A) It almost always accompanies alpha or beta emission.
B) It is a mechanism to release excess energy in the nucleus.
C) Gamma rays are high energy photons.
D) The mass number decreases by one with each gamma emitted.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the periodic table below to answer the following questions. 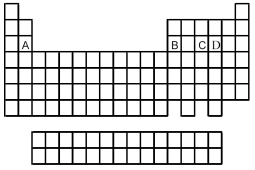 -Which is the correct formula of the binary fluoride of element A?
-Which is the correct formula of the binary fluoride of element A?
A) AF2
B) AF3
C) AF5
D) AF6
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In addition to a beta particle,what is the other product of beta decay of 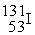 ?
?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a part of Dalton's atomic theory?
A) Atoms are rearranged but not changed during a chemical reaction.
B) Atoms break down during radioactive decay.
C) Atoms contain protons,neutrons,and electrons.
D) Isotopes of the same element have different masses.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the periodic table below to answer the following questions. 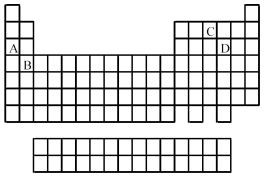 -Which elements commonly form anions?
-Which elements commonly form anions?
A) A and B
B) A and C
C) B and D
D) C and D
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element can form more than one kind of monatomic ion?
A) Ca
B) Cl
C) Cr
D) Cs
F) A) and C)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
A sample of pure calcium fluoride with a mass of 15.0 g contains 7.70 g of calcium.How much calcium is contained in 45.0 g of calcium fluoride?
A) 2) 56 g
B) 7) 70 g
C) 15.0 g
D) 23.1 g
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Gold is an example of
A) a compound.
B) an element.
C) a heterogeneous mixture.
D) a homogeneous mixture.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The observation that hydrogen and oxygen can react to form two compounds with different chemical and physical properties,one having an O:H mass ratio = 8:1 and the other having an O:H mass ratio = 16:1 is consistent with the law of
A) definite proportions.
B) energy conservation.
C) mass conservation.
D) multiple proportions.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 257
Related Exams